Polycythemia Vera
;;;;↾Read more at https://www.brainyquote.com/topics/cancer-quotesMy gold, my money couldn't stop cancer from appearing on my body. If they can't save me, then I don't need them.
Mr. T
Read more at https://www.brainyquote.com/topics/cancer-quotes
My gold, my money couldn't stop cancer from appearing on my body. If they can't save me, then I don't need them.~ Mr. T
Cancer is like the common cold; there are so many different types. In the future we'll still have cancer, but we'll detect it very, very early, so that it won't kill anybody. We'll zap it at the molecular level decades before it grows into a tumor.
~ Michio Kaku
Symptoms vary among individuals, but can include: fatigue, headache, itching, dizziness, vision problems, night sweats, enlarged spleen, fevers, joint pain, and a host of others. Polycythemia vera isn't common, although some think that it is underdiagnosed. PV is one of a group of blood cancers known as myeloproliferative neoplasms (MPNs). These are a group of bone marrow diseases in which an excess of certain types of blood cells are produced. Other diseases in this group include essential thrombocythemia (ET) and primary myelofibrosis (PMF). Over time, there may be a risk of progressing to more-serious blood cancers, such as acute myeloid leukemia (AML). The common biological phenomena involved in these diseases involves dysregulation of key molecular signals. Three key genomic mutations have been identified in MPNs - mutations in the JAK2, CALR, TET2, or MPL genes. These mutations constitutively activate the JAK-STAT pathway in haematopoietic stem cells. The JAK2 V617F mutation is considered definitive in the diagnosis of PV, occurring in approximately 99% of cases.
Jak2 is strongly conserved across mammals. This figure shows the amino acid alignment of eight mammals: human, mouse, rat, cow, rabbit, dog, rhesus monkey, and chimp.
 |
| Eight mammalian Jak2 proteins |
Members of the Jak family, Jak1, 2, 3, and TYK2 are also strongly conserved.
 |
| Jak family alignment |
JAK2 V617F Mutation
Janus kinase 2 (JAK2) is a tyrosine kinase. It is involved in signaling by type II cytokine receptor family members, GM-CSF family members, and others. Non-receptor tyrosine kinases (nRTKs) are cytosolic enzymes that are responsible for catalysing the transfer of a phosphate group from a nucleoside triphosphate donor, such as ATP, to tyrosine residues in proteins. Type I cytokine receptors, such as receptors for erythropoietin (Epo), thrombopoietin (Tpo), granulocyte colony stimulating factor (G-CSF) and most interleukins (IL) receptors, and type II interferons (IFN) lack intrinsic catalytic activity. In order to initiate downstream signaling these receptors bind one or several members of cytoplasmic tyrosine kinases of the Janus kinase (JAK) family. (See https://www.tandfonline.com/doi/full/10.4161/jkst.22071)
A common feature of PV is the acquired V617F mutation in the JAK2 gene. This mutation is thought to be causative for MPNs.JAK2 is located at 9p24.1, which is the short (p) arm of chromosome 9 at position 24.1. The molecular location is base pairs 4,985,086 to 5,128,183 on chromosome 9 (Homo sapiens Updated Annotation Release 109.20190905, GRCh38.p13)
 |
| https://ghr.nlm.nih.gov/gene/JAK2#location |
The following figure shows a PyMOL rendering of Jak2 (PDB ID: 2b7a).
The V617F is a change of a valine to a phenylaline at position 617 on the coding DNA reference sequence. The coding DNA reference sequence refers to a cDNA-derived sequence containing the full length of all coding regions and noncoding untranslated regions. See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1867422/. The mutation is a substitution of an Adenine (A) for a Cytosine (C) in the codon: CAG => AAG.
The Jak protein family possess an N-terminal FERM domain, a Src homology-2 (SH2)-like domain, a pseudokinase domain (Jak homology-2, JH2), and a C-terminal tyrosine kinase domain (JH1). JH1 is activated via trans-phosphorylation of tandem tyrosines in the activation loop (Tyr1007 and Tyr1008 in Jak2). JH2 regulates the activity of JH1. The JH2 pseudokinase lacks key residues needed for kinase activity. The V617F mutation is in the JH2 domain. Mutations in JH2 of Jak1–3 genes have also been linked to leukemias, such as acute lymphoblastic leukemia and acute myeloid leukemia. All of these mutations result in constitutive tyrosine kinase activity of Jak2. See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3414675/pdf/nihms389422.pdf
 |
| https://en.wikipedia.org/wiki/Janus_kinase_2#/media/File:Protein_JAK2_PDB_2b7a.png |
The V617F is a change of a valine to a phenylaline at position 617 on the coding DNA reference sequence. The coding DNA reference sequence refers to a cDNA-derived sequence containing the full length of all coding regions and noncoding untranslated regions. See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1867422/. The mutation is a substitution of an Adenine (A) for a Cytosine (C) in the codon: CAG => AAG.
The Jak protein family possess an N-terminal FERM domain, a Src homology-2 (SH2)-like domain, a pseudokinase domain (Jak homology-2, JH2), and a C-terminal tyrosine kinase domain (JH1). JH1 is activated via trans-phosphorylation of tandem tyrosines in the activation loop (Tyr1007 and Tyr1008 in Jak2). JH2 regulates the activity of JH1. The JH2 pseudokinase lacks key residues needed for kinase activity. The V617F mutation is in the JH2 domain. Mutations in JH2 of Jak1–3 genes have also been linked to leukemias, such as acute lymphoblastic leukemia and acute myeloid leukemia. All of these mutations result in constitutive tyrosine kinase activity of Jak2. See https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3414675/pdf/nihms389422.pdf
The following figure from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3414675/pdf/nihms389422.pdf (Figure 3a) compares the structure of JH2 with the V617F mutation (JH2-VF) to the wild type JH2 domain (JH2-WT). JH2-WT alpha-C is colored yellow.. JH2-VF is colored pink. The side chains of Phe594, Phe595 and Val or Phe617 are shown in stick representation. The N-terminus (residue 536) is labeled N. The catalytic loop (C loop), the activation loop (A loop), αC and other select segments are labeled
 |
| https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3414675/pdf/nihms389422.pdf |
The effect of the V617F mutation either releases the inhibitory function of the JH2 domain on the JAK2 catalytic domain, or that it induces a conformational change to the JH2 pseudokinase domain that in turn activates the kinase domain. In either case, STAT proteins, especially STAT5 and STAT3,become constitutively activated. The JAK2 V617F mutant induces several downstream signaling pathways, recapitulating in a persistent manner the pathways transiently activated by cytokines, i.e., STAT5/3, MAP-kinase,PI-3'-kinase/Akt and mTOR pathways. Interestingly, there are differences in STAT signaling between different MPNs. PV patients exhibit high STAT5 and STAT3 phosphorylation, while ET patients exhibit high STAT3 but low STAT5 phosphorylation. Myelofibrosis patients exhibit low STAT5 and low STAT3 phosphorylation. Several lines of evidence indicate that constitutive activation of JAK2-STAT5 and STAT3 signaling is the basis of PV, ET and PMF. https://www.tandfonline.com/doi/pdf/10.4161/jkst.22071?needAccess=true The dimerized STAT proteins are transfered to the nucleus where they modify gene expression to transform hematopoietic stem cells to common myeloidprogenitor (CMP) cells, eventually forming blood cells.
The figure below shows the putative effect the V617F mutation. With wild type Jak2, the binding of a ligand such as erythropoietin to the extra-cellular domain induces a conformational change of the cytokine receptor and allows transphosphorylation of the protein. Activated JAKs phosphorylate tyrosine residues in the receptor cytoplasmic domain and provide a docking site for STAT proteins. Phosphorylated STATs dissociate from the receptor, dimerize and translocate to the nucleus where they modulate gene expression. In the case of the mutated Jak2 protein, mutant cytosolic tyrosines are constantly phosphorylated, leading to constitutive activation of STAT proteins. https://www.tandfonline.com/doi/full/10.4161/jkst.22071
 |
| https://www.tandfonline.com/doi/pdf/10.4161/jkst.22071?needAccess=true |
 |
| https://en.wikipedia.org/wiki/STAT3 |
CALR Mutations
Mutations in the calreticulin (CALR) gene occur in approximately 20% of patients with MPNs. CALR is a chaperone protein whose main function is to bind to misfolded proteins and prevents them from being exported from the endoplasmic reticulum to the Golgi apparatus. Mutations in the gene encoding CALR occurred in the majority of non-JAK2/MPL mutated ET and PMF patients. The two most common mutations are a 52 base-pair deletion (L367fs*46) and a 5 base-pair insertion (K385fs*47). The effect of these mutations is ligand-independent JAK/STAT signaling activation. https://www.ncbi.nlm.nih.gov/pubmed/31562135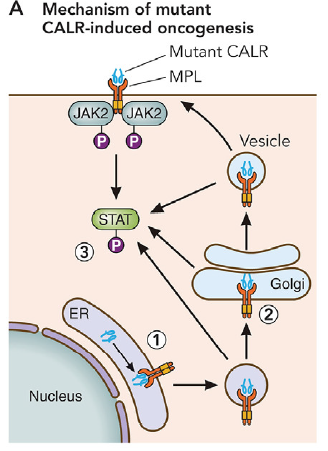 |
| https://www.ncbi.nlm.nih.gov/pubmed/31562135 |
The following figure shows the structure of the non-mutated CALR protein/
 |
| https://en.wikipedia.org/wiki/Calreticulin |
TET2 Mutations
The TET2 gene provides instructions for making a protein whose function is unknown. It is assumed that it is a transcription regulator. Mutations in this gene have been found in approximately 16 percent of people with PV. It is unclear what role these mutations play in the development of polycythemia vera. https://ghr.nlm.nih.gov/gene/TET2#conditions. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4654629/ shows a ollection of TET2 mutations in 32 patients with MPNs.


Polycythemia is a long-running situation and does not have any procedure of treatment or says it can’t be cured. Though, specialists are of the outlook that intake of Natural Remedies for Polycythemia Vera could assist in thinning of blood and obstruct blood clots.
ReplyDelete